It is essential that the soil contain all the elements found in the plant itself, and that it retain them long enough for the plant to absorb them during its life. The chemical elements most needed by plants and most liable to rapid exhaustion are nitrogen, phosphoric acid (phosphorus), and potash (potassium). (This is the classic N-P-K ratio you see on every container or bag of commercial fertilizer.)
There are, of course, other chemical elements, but they are used in such small quantities that they are lost from the soil only in exceptional cases. The elements are used by the plant only in solution.
Acid vs. Alkaline
Acidity and alkalinity of the soil also affect fertility and can be the determining factor as to which kinds of plants to grow.
As acid soil exists when there are more hydrogen ions in a mixed soil and water sample than hydroxyl ions. A soil in neutral when the soil-water mixture contains an equal number of both hydrogen ions (acid) and hydroxyl ions (alkaline). This all makes perfect sense to the soil scientist, but the gardener need only concern himself with the pH scale, a system invented to express the acidity or alkalinity of solutions, such as soil-water solution.
Fortunately, most plants thrive in a slightly acid to neutral or slightly alkaline soil. While sometimes the degree of acidity or alkalinity of soil can be the cause of poor growth or plant failure, it is wrong to assume that an application of limestone may correct the trouble. The soil should first be tested by the home gardener, using one of the kits available from garden centers or mail-order nurseries, or by a professional (consult your county agent or State agricultural school outreach). The results of the test, expressed in the terms of the pH scale , indicate how much lime must be added to raise the pH.
While too much lime can be harmful to soils and plants, there are definite benefits it can give in addition to affecting the pH of the soil. It supplies calcium, makes porous soils more compact, and increases the activity of beneficial soil bacteria.
In addition to testing for acidity, it is possible, by applying certain chemicals to a small amount of soil (or to water allowed to leach through it), and noting the reaction, to determine the amounts of the important plant food elements in the soil To be really accurate and of most value, such tests should be made in a laboratory with complicated apparatus, and the results should be studied in connection with a careful examination of the physical condition of the land, the crops it bears, and its management. State agricultural experiment stations and sometimes county agents will make such tests. For gardeners there are inexpensive kits with detailed instructions.
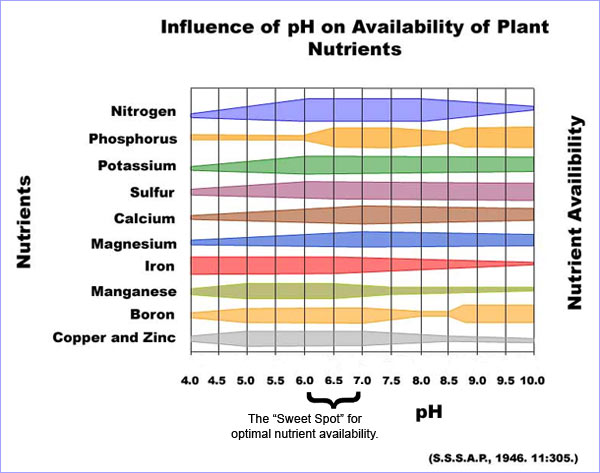 A very clear chart showing how the availability of nutrients change with a change in soil pH. Even though key nutrients are IN the soil, the pH has to be at the right level to make it "available" for plants to take in.
A very clear chart showing how the availability of nutrients change with a change in soil pH. Even though key nutrients are IN the soil, the pH has to be at the right level to make it "available" for plants to take in.
The Nature of Soil
Soil is composed of five main ingredients:
- mineral particles
- humus
- micro flora and fauna
- air
- water
The mechanical characteristics of soil (its texture) are determined mainly by the size of the mineral particles.
The relative fertility is determined partly by the origin of the mineral particles and by the amount of humus present. The amount of humus also largely determines the relative populations of micro flora and fauna.
The amount of air in soil is determined by a combination of mineral particle size, humus and micro flora and fauna. Of these three factors, the mineral particle size is probably the most important.
The amount of water passing through soil is determined mainly by the mineral particle size. The amount of water retained by soil is determined mainly by the amount of humus (or organic matter) present.
None of these five factors is of undue importance on its own. What really matters is the relative proportions in which they are combined.
The Origins of Mineral Particles
The mineral particles in soil originate from the basic rocks of which the earth's crust is formed. The fertility of soil is directly related to the type of rock from which the mineral particles originally came. Over many years these rocks have been broken down into smaller and smaller fragments by extreme climatic conditions such as glaciation. The erosion of rocks is still going on under the action of wind, rain, sun, ice, and other agents such as lichen and mosses.
The Function of Humus
Animal and vegetable remains are gradually broken down by the weather and by soil organisms. Humus is the substance which remains after the partial breakdown of this organic matter. It is a highly complex, microscopic, brown and black, colloidal material containing fats, carbohydrates and proteins.
Humus is important to the health of both soil and plants in four ways:
1) It acts as a store for plant foods which tend to adhere very tightly to the surface of humus particles, making them less likely to be lost from the soil through drainage. Humus is the only soil store for nitrogen.
2) It binds mineral particles together, preventing their erosion by wind and water and makes clay soils easier to work.
3) It is a reservoir for soil moisture but also helps improve drainage and passage of air through the soil.
4) It provides a source of food for soil flora and fauna which in itself is essential to healthy plant growth.
Many soils can be greatly improved by increasing the amount of humus present.
Microscopic Flora and Fauna
 Rhizobia bacteria in the soil cause nodules to grow on
Rhizobia bacteria in the soil cause nodules to grow onleguminous plant roots. This is where inert nitrogen
from the air is converted to a form of nitrogen
the plant can utilize. Image from Wikipedia.
A rich and varied soil microorganism population is highly desirable. Microorganisms thrive under exactly the same conditions as the roots of plants thrive; further, they compete for the same nutrients. In any soil which contains plenty of organic matter and has a rich microorganism population, the microorganisms continually compete with each other. This prevents any one specialized disease organism or fungus from gaining ascendency. In the course of their activities, the soil organisms make many nutrients available to plants in a form in which the plants can readily use (nitrogen fixing bacteria are a well-studied example of this).
The Layers of Soil
Soil is an infinitely variable material: there is no chemical formula for it, and no two samples, even from the same garden, will be exactly similar. However, in most gardens two distinct layers of soil will be found. The first layer is the fertile top soil, which is usually dark in color. Below this is the infertile subsoil which is usually lighter in color.
Gardeners are concerned with the top soil - this is where plants have their feeding roots. The anchoring roots of plants may penetrate the subsoil, but since it is generally infertile it should never be brought to the surface. When gardeners speak of soil they're usually referring to just the top soil.
Resources
What's Soil pH and Why Do I Care? by Rachel Gilker at On Pasture.
All about Rhizobia soil bacteria at Wikipedia.